Biospecimen Pre-analytical Variables (BPV) Program
The National Cancer Institute’s Biorepositories and Biospecimen Research Branch (BBRB) Biospecimen Pre-analytical Variables Program rigorously investigated variables affecting the integrity of biospecimens prior to their analysis. The ultimate goal was to develop evidence-based protocols and best practices for the collection, processing and storage of biospecimens, thereby enhancing the field’s standards and adding confidence to research results.
A large number of highly annotated biospecimens were collected following well-defined protocols, including tumor tissue and blood from kidney, ovary, lung and colon cancer patients, which were used to assess the impact of various pre-analytical factors on the molecular integrity of biospecimens. Pre-analytical factors examined included delay to fixation (DTF) and time in fixative (TIF) for FFPE tissues (see table 1). Additionally, freezing methods and/or storage temperature variations were studied for tissue and plasma.
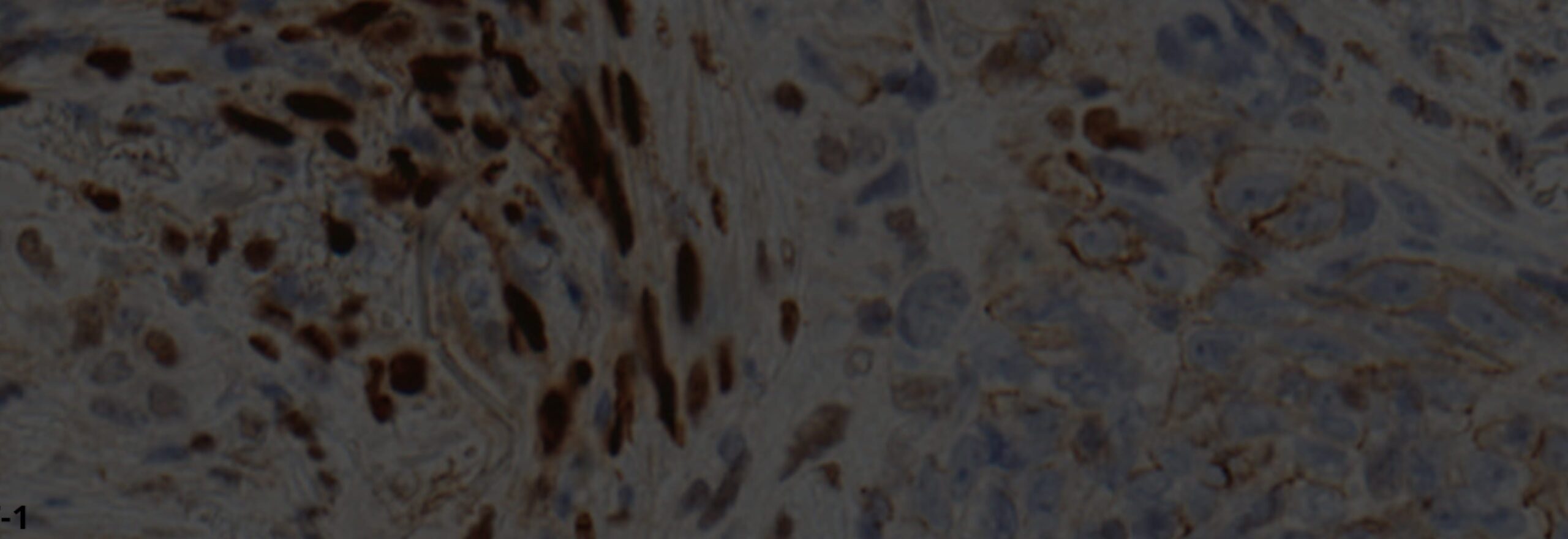
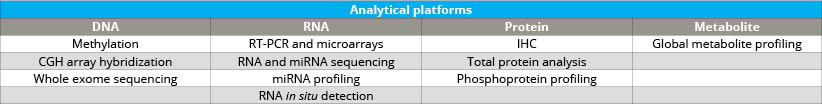
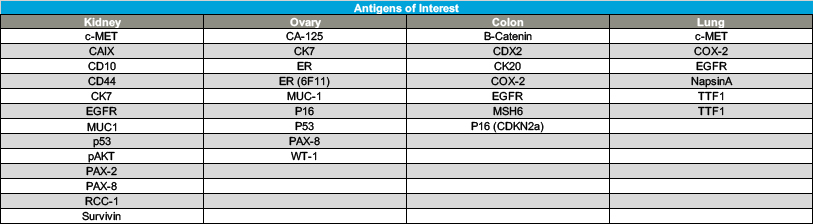
As the Comprehensive Biospecimen Resource for this program, Van Andel Institute’s Biorepository compiled and shipped collection kits to sites across the country. Upon return to the Biorepository, these samples were accessioned, processed, inventoried and stored. The specimens also were assessed for histological quality and were analyzed by next-gen sequencing, miRNA assay, immunohistochemistry and mass spectrometry (see tables 2 and 3).
Collection has ended and final study analysis can be found here. A list of current publications based on this project can be found here. A large number of specimens remain available for research use through a collaboration agreement with the NCI BBRB. Through this collaboration, the VAI PBC continues to store, maintain and distribute these biospecimens. To download and view the BPV biospecimen inventory, please click the button below. To request access to this collection, please click the button below.
View Inventory Request Access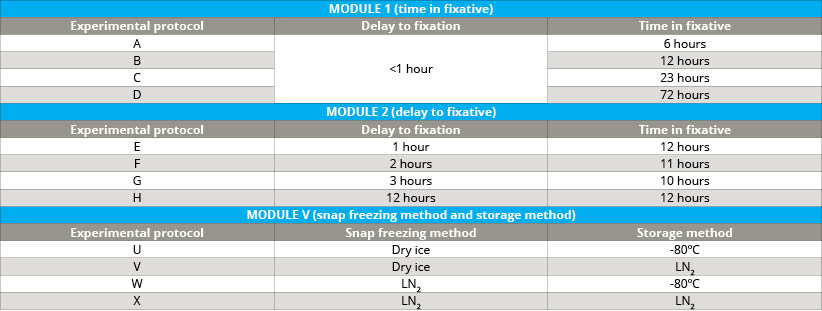
Tissue Types Collected
Along with the samples, matched blood was collected as well as normal adjacent tissue, when possible.
- Renal cell carcinoma
- Ovarian, fallopian tube and primary peritoneal carcinoma
- Lung adenocarcinoma
- Lung squamous cell carcinoma
- Colorectal adenocarcinoma
Specimen Availability
Following surgical removal, tumors were dissected into six blocks—one was frozen for use in future research, a second block was formalin-fixed and paraffin-embedded for quality control and an H&E slide was generated, and the remaining four were randomly assigned to an experimental protocol.
The H&E section taken from the QC block was reviewed by a pathologist for confirmation of diagnosis and to ensure the requirement of at least 50% tumor cells by surface area and less than 20% necrosis was met. The samples were used to evaluate the effects of pre-analytical factors on downstream molecular analysis results, such as:
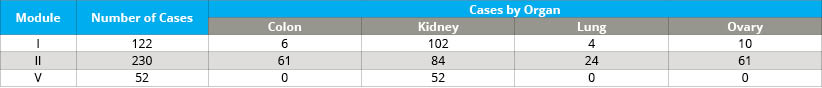
- Cold ischemia time of FFPE tumor tissue (delay to fixation; module I)
- Incubation time in formalin of FFPE tumor tissue (time in fixative; module II)
- Method of freezing tumor tissue (module V)
- Storage temperature of frozen tumor tissue (module V)
- Frozen storage temperature of plasma (module V)
For a list of tissue types, please see Table 4. The BPV collection retains thousands of samples that are available for research requests (see Table 5).
Tissue microarrays (TMAs) also are available for each tissue type collected. All TMAs were created using duplicate 1.0 mm cores randomly placed in each TMA block. Three control tissues were included in each TMA. The TMAs represent modules I and II. Except for kidney, the modules are randomized throughout the TMAs. Each TMA was created in duplicate. A total of 293 patients are represented across these 21 TMAs. Available TMAs include the following:
Colon TMAs: 4 Colon TMAs (plus 4 duplicates), 56 patients
- TMA 1 = 114 cores, 57 blocks, 12 patients
- TMA 2 = 114 cores, 57 blocks, 13 patients
- TMA 3 = 118 cores, 60 blocks, 15 patients
- TMA 4 = 97 cores, 49 blocks, 16 patients
Kidney TMAs: 11 Kidney TMAs (plus 11 duplicates), 165 patients
- Module I TMA 1 = 120 cores, 60 blocks, 12 patients
- Module I TMA 2 = 120 cores, 60 blocks, 14 patients
- Module I TMA 3 = 119 cores, 60 blocks, 15 patients
- Module I TMA 4 = 118 cores, 60 blocks, 15 patients
- Module I TMA 5 = 120 cores, 60 blocks, 15 patients
- Module I TMA 6 = 115 cores, 58 blocks, 22 patients
- Module II TMA 1 = 115 cores, 58 blocks, 12 patients
- Module II TMA 2 = 115 cores, 58 blocks, 13 patients
- Module II TMA 3 = 115 cores, 55 blocks, 15 patients
- Module II TMA 4 = 115 cores, 59 blocks, 15 patients
- Module II TMA 5 = 115 cores, 51 blocks, 17 patients
Lung TMAs: 2 Lung TMAs (plus 2 duplicates), 20 patients
- TMA 1 = 108 cores, 54 blocks, 12 patients
- TMA 2 = 102 cores, 51 blocks, 8 patients
Ovary TMAs: 4 Ovary TMAs (plus 4 duplicates), 52 patients
- TMA 1 = 120 cores, 60 blocks, 14 patients
- TMA 2 = 117 cores, 60 blocks, 15 patients
- TMA 3 = 96 cores, 48 blocks, 14 patients
- TMA 4 = 64 cores, 32 blocks, 9 patients
